Citric acid

Citric acid is a compound present in various biological fluids and is involved in numerous metabolic processes essential for the functioning of the human body. Its presence and concentration in various biological fluids can provide useful information on the health and physiological state of the individual.
The concentration of citric acid in urine is a useful indicator for several reasons:
- Prevention of kidney stone formation: Citric acid has an inhibiting effect on the formation of kidney stones, especially those containing calcium. Citrate in urine binds calcium, preventing it from combining with other substances (such as oxalate and phosphate) and forming crystals that could evolve into stones. A low level of citric acid in urine can increase the risk of kidney stones.
It should be noted that citrate does not “dissolve” calcium oxalate and calcium phosphate stones and, to date, there are no therapies capable of dissolving these stones, but adequate levels of citrate in urine decrease both the possibility of an already formed stone increasing in volume and the formation of new calcium microcrystals. - Indicator of kidney function: alterations in urinary levels can indicate problems such as kidney dysfunction.
- Acid-Base Balance and Metabolism: Citric acid, as part of the citric acid cycle, helps maintain the acid-base balance in the body. Low urine citrate levels may be a sign of an impact on metabolism or other metabolic conditions, such as acidosis.
- Diet and Lifestyle: Diets high in foods that increase citric acid excretion (such as fruits and vegetables) can lead to higher levels in the urine, while other conditions, such as dehydration or insufficient fluid intake, can reduce urinary citrate concentration.
Monitoring urine citric acid levels is important for preventing kidney stones, understanding the body’s acid-base balance, and monitoring kidney health. Low urine citric acid concentration may be a sign of increased risk for kidney disease or other metabolic imbalances.
Citric acid is also present in semen in significant concentrations and is secreted by the prostate. The presence and levels of citric acid in semen are useful indicators for various aspects of male reproductive health.
- Prostate function: Altered levels of citric acid may suggest prostate dysfunction, such as inflammation (prostatitis) or other pathologies (such as benign prostatic hyperplasia or prostate cancer).
- Semen pH: Citric acid helps to maintain the pH of semen. A balanced pH is essential for sperm motility and vitality.
- Support for sperm mobility: Citric acid plays a role in providing energy to sperm, as it is involved in cellular metabolism through the citric acid cycle (Krebs cycle). This energy is necessary for sperm motility and vitality.
- Indicator of semen quality: Lower levels of citric acid may be associated with low sperm production or prostate dysfunction, while adequate levels indicate of normal prostate function.
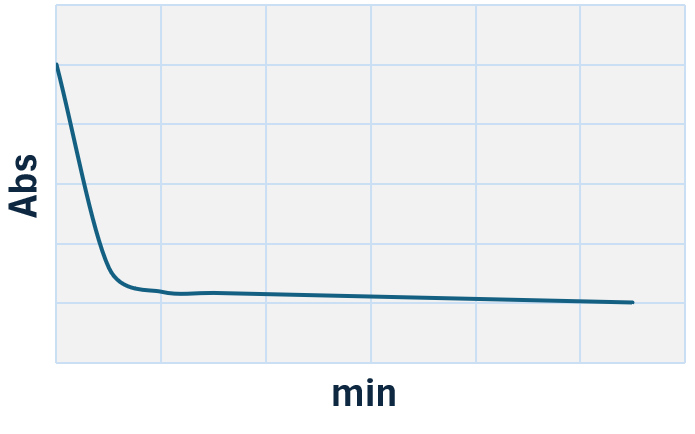
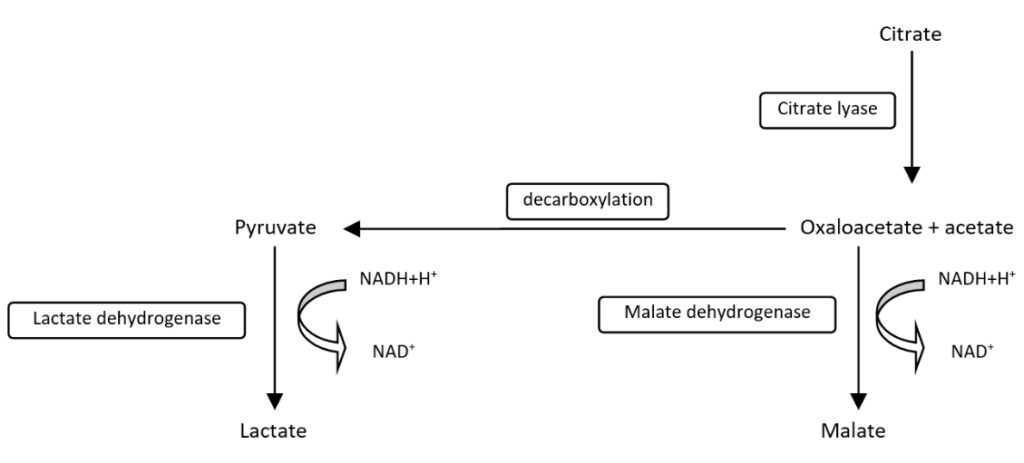
LTA test is a UV enzymatic determination with Citrate Lyase method.
Citric acid in the sample (citrate) is converted into oxaloacetate and acetate by Citrate Lyase (CL). In the presence of Malate Dehydrogenase (MDH) and Lactate Dehydrogenase (LDH), oxaloacetate and pyruvate, the decarboxylation product of oxaloacetate, are reduced to L-malate and L-lactate, respectively, causing the oxidation of NADH to NAD+. The reaction is kinetically monitored at 340 nm by the decrease in absorbance resulting from the oxidation of NADH to NAD+, proportional to the activity of citric acid in the sample. Sensitivity 0.02 g/L. Linearity up to 2 g/L.
Package contents:
- Buffer 1 x 100 mL
- Substratum 5 x 20 mL (lyophilized)
- Starter 5 x 0,5 mL (lyophilized)
- Standard 0,25 g/L 1 x 5 mL
**Samples Diluent 2 x 65 mL
(only in the version for seminal fluid)

